Perspective on SARS-CoV-2 Detection and Reference Materials
-
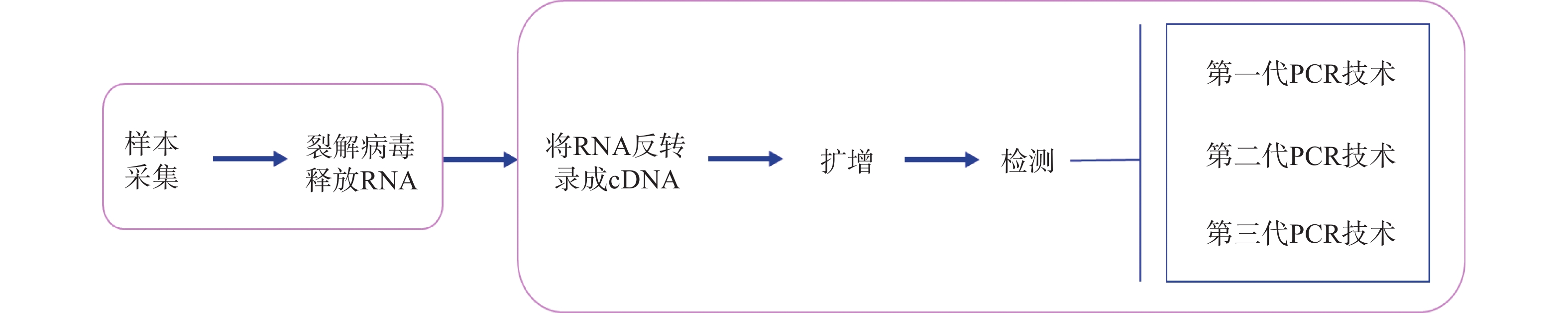
摘要: 新型冠状病毒(SARS-CoV-2)具有很强的传染能力,已造成全球大流行,对人类健康和社会的经济发展造成极大影响。快速、准确的检测新型冠状病毒对病情的确诊和疫情的防控尤为重要。我国国家市场监督管理总局已注册22种新型冠状病毒核酸检测试剂盒,能满足对高危人群的大规模筛查,有力保障疫情防控。同时,中国计量科学研究院研制的新型冠状病毒标准物质作为计量实体,进一步保障检测结果的准确性和溯源性。通过对国家食品药品监督管理局和国家标准物质资源共享平台公布的数据进行分析和梳理,结合国内外相关研究进展,就新型冠状病毒的检测方法、检测试剂盒及标准物质进行综述,以期为新型冠状病毒及其他传染性疾病的诊断和防控提供借鉴和参考思路。Abstract: The novel coronavirus (SARS-CoV-2) has a strong ability to infect, which has caused the pandemic. It has seriously affected human health and life, social economy and development. Fast and accurate detection of the novel coronavirus is particularly important for the diagnosis, prevention, and control of the epidemic. Chinese CFDA has registered 22 nucleic acid detection kits, which can meet the needs of high-risk groups for large-scale screening and effectively guarantee the prevention and control of the epidemic. Meanwhile, the novel coronavirus reference material, as a measurement reference, developed by the National Institute of Metrology, China further guarantees the accuracy and traceability of test results. This article analyzes the data published by the State Food and Drug Administration and the National Standard Material Sharing Platform, and combines relevant domestic and international research results to systematically review the novel coronavirus detection methods, test kits and reference materials, hoping to shed light on diagnosis, prevention and control of the novel coronavirus and other infectious diseases.
-
Key words:
- metrology /
- novel coronavirus /
- in vitro diagnosis /
- nucleic acid detection /
- reference materials
-
表 1 CFDA注册的新型冠状病毒核酸检测试剂盒
Table 1. The registration SARS-CoV-2 detection kit of CFDA
序号 名称 注册证编号 适用范围 1 新型冠状病毒2019-nCoV核酸分析软件 20203210062 核酸检测分析软件 2 新型冠状病毒2019-nCoV核酸检测试剂盒(荧光PCR法) 20203400520 ORF1ab和N基因 3 新型冠状病毒(2019-nCoV)核酸检测试剂盒(荧光PCR法) 20203400299 ORF1ab和N基因、E基因 4 新型冠状病毒2019-nCoV核酸检测试剂盒(双扩增法) 20203400302 ORF1ab和E基因 5 新型冠状病毒2019-nCoV核酸检测试剂盒(荧光PCR法) 20203400322 ORF1ab基因、N基因 6 新型冠状病毒2019-nCoV核酸检测试剂盒(荧光PCR法) 20203400384 ORF1ab和N基因 7 新型冠状病毒2019-nCoV核酸检测试剂盒(荧光PCR法) 20203400065 ORF1ab和N基因 8 新型冠状病毒2019-nCoV核酸检测试剂盒(荧光PCR法) 20203400057 ORF1ab和N基因、E基因 9 新型冠状病毒2019-nCoV核酸检测试剂盒(荧光PCR法) 20203400060 ORF1ab基因 10 新型冠状病毒2019-nCoV核酸检测试剂盒(荧光PCR法) 20203400184 ORF1ab、N基因和E基因 11 新型冠状病毒2019-nCoV核酸检测试剂盒(荧光PCR法) 20203400058 ORF1ab和N基因 12 新型冠状病毒2019-nCoV核酸检测试剂盒(荧光PCR法) 20203400644 ORF1ab和N 基因 13 新型冠状病毒2019-nCoV核酸检测试剂盒(荧光PCR法) 20203400064 ORF1ab和N基因 14 新型冠状病毒2019-nCoV核酸检测试剂盒(荧光PCR法) 20203400063 ORF1ab和N基因 15 新型冠状病毒2019-nCoV核酸检测试剂盒(荧光PCR法) 20203400537 ORF1ab基因和N基因 16 新型冠状病毒2019-nCoV核酸检测试剂盒(荧光PCR法) 20203400179 ORF1ab、N基因和E基因 17 新型冠状病毒2019-nCoV核酸检测试剂盒(荧光PCR法) 20203400535 ORF1ab基因 18 新型冠状病毒2019-nCoV核酸检测试剂盒(荧光PCR法) 20203400212 ORF1ab基因、N基因 19 新型冠状病毒2019-nCoV核酸检测试剂盒(RNA捕获探针法) 20203400300 ORF1ab基因 20 新型冠状病毒2019-nCoV核酸检测试剂盒(RNA恒温扩增-金探针层析法) 20203400301 ORF1ab和E基因 21 新型冠状病毒2019-nCoV核酸检测试剂盒(联合探针锚定聚合测序法) 20203400059 核酸序列 22 新型冠状病毒2019-nCoV核酸检测试剂盒(恒温扩增-实时荧光法) 20203400241 ORF1ab基因、N基因 表 2 新型冠状病毒的国家有证核酸标准物质
Table 2. Certified nucleic acid reference materials of SARS-CoV-2
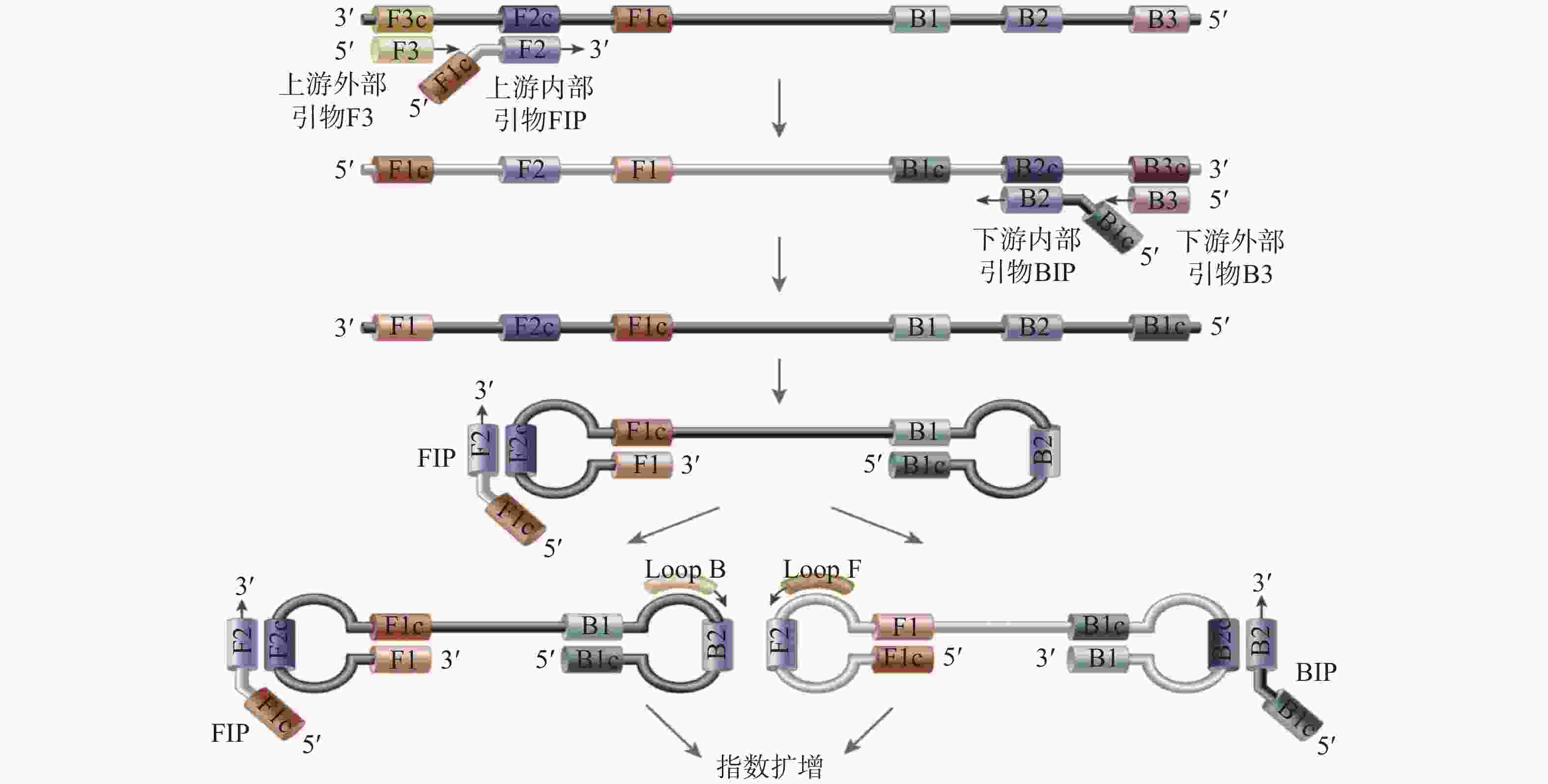
序号 标物名称 编号 标准值 定值方法 1 新型冠状病毒核酸标准物质(高浓度) GBW(E)091089 E基因 (7.05±0.54)×105 copy/μL ORF1ab (1.10±0.12)×106 copy/μL N基因 (8.38±0.76)×105 copy/μL 2 新型冠状病毒核酸标准物质(低浓度) GBW(E)091090 E基因 (7.63±0.66)×102 copy/μL ORF1ab (1.24±0.19)×103 copy/μL N基因 (9.8±1.2)×102 copy/μL 3 新型冠状病毒核糖核酸基因组标准物质 GBW(E)091098 E基因 (1.29±0.26)×102 copy/μL ORF1ab (9.5±1.8)×103 copy/μL N基因 (2.05±0.31)×102 copy/μL 4 新型冠状病毒核糖核酸基因组标准物质 GBW(E)091099 E基因 (1.06±0.11)×103 copy/μL 数字PCR ORF1ab (8.96±0.61)×102 copy/μL N基因 (1.73±0.13)×103 copy/μL 5 新型冠状病毒体外转录RNA标准物质 GBW(E)091111 E基因 (2.0±0.3)×105 copy/μL ORF1ab (1.2±0.2)×105 copy/μL N基因 (1.0±0.1)×106 copy/μL 6 新型冠状病毒体外转录RNA标准物质 GBW(E)091112 E基因 (2.0±0.3)×107 copy/μL ORF1ab (1.2±0.2)×107 copy/μL N基因 (1.0±0.1)×108 copy/μL 7 新型冠状病毒(2019-nCoV)假病毒核糖核酸标准物质 NIM-RM5203 E基因 (4.6±0.9)×102 copy/μL ORF1ab (6.5±1.2)×102 copy/μL N基因 (4.3±0.9)×102 copy/μL -
[1] Zhou P, Yang X L, Wang X G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin[J]. Nature, 2020, 579(7798): 270-273. doi: 10.1038/s41586-020-2012-7 [2] Kannan S, Ali P S S, Sheeza A, et al. COVID-19 (Novel Coronavirus 2019)-recent trends[J]. European Review for Medical and Pharmacological Sciences, 2020, 24: 2006-2011. [3] Mustafa H, Kili S, Sara F. Coronaviruses and SARS-CoV-2[J]. Turkish Journal of Medical ences, 2020, 50: 1. [4] Watson J D, Crick F H. Molecular structure of nucleic acids: a structure for deoxyribose nucleic acid[J]. Nature, 1974, 248(5451): 765. doi: 10.1038/248765a0 [5] Ciotti M, Angeletti S, Minieri M, et al. COVID-19 Outbreak: An Overview[J]. Chemotherapy, 2020, 64(5-6): 1-9. [6] Pai-dhungat J. Kary mullis-inventor of PCR[J]. J. Assoc. Physicians. India., 2019, 67(9): 96. [7] Kazuyuki M. PCR-based detection methods for single-nucleotide polymorphism or mutation: real-time pcr and its substantial contribution toward technological refinement[J]. Adv. Clin. Chem. 2017, 80: 45-72. [8] Beata K, Józef K, Karolina S, et al. Principles and applications of ligation mediated pcr methods for dna-based typing of microbial organisms[J]. Acta. Biochim. Pol. 2016, 63(1): 39-52. [9] Dahiya B, Mehta P K. Detection of potential biomarkers associated with outrageous diseases and environmental pollutants by nanoparticle-based immuno-PCR assays[J]. Anal. Biochem., 2019, 587: 113444. doi: 10.1016/j.ab.2019.113444 [10] Kakizawa S. A multiplex-pcr method for diagnosis of ay-group phytoplasmas[J]. Methods Mol. Biol, 2019, 1875: 143-149. [11] Rajesh K, Sinha R P. Real-time immuno-PCR: an approach for detection of trace amounts of transgenic proteins[J]. Journal of Aoac Internationalm, 2014, 97(6): 1634-1637. doi: 10.5740/jaoacint.14-044 [12] Nemeth A, Wurz A, Artim L, et al. Sensitive PCR analysis of animal tissue samples for fragments of endogenous and transgenic plant DNA[J]. Journal of Agricultural & Food Chemistry, 2004, 52(20): 6129. [13] Ehnert S, Linnemann C, Braun B, et al. One-step arms-pcr for the detection of snps-using the example of the padi4 gene[J]. Methods Protoc, 2019, 2(3): 63. doi: 10.3390/mps2030063 [14] Romsos E L, Vallone P M. Rapid pcr of str markers: applications to human identification[J]. Forensic. Sci. Int. Genet, 2015, 18: 90-99. doi: 10.1016/j.fsigen.2015.04.008 [15] 张永卓, 王 晶, 傅博强, 等. 2019新型冠状病毒的核酸检测[J]. 计量学报, 2020, 41(4): 393-398. doi: 10.3969/j.issn.1000-1158.2020.04.01 [16] Wong Y P, Othman S, Lau Y L, et al. Loop-mediated isothermal amplification (LAMP): a versatile technique for detection of micro-organisms[J]. Journal of Applied Microbiology, 2018, 124(3): 626-643. doi: 10.1111/jam.13647 [17] Notomi T, Okayama H, Masubuchi H, et al. Loop-mediated isothermal amplification of DNA[J]. Nucleic acid Res, 2000, 28(12): E63. doi: 10.1093/nar/28.12.e63 [18] Mori Y, Nagamine K, Tomita N, et al. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation[J]. Biochem Biophys Res Commun, 2001, 289(1): 150-154. doi: 10.1006/bbrc.2001.5921 [19] Blomstrom A L, Hakhverdyan M, Reid S M, et al. A one-step reverse transcriptase loop-mediated isothermal amplification assay for simple and rapid detection of swine vesicular disease virus[J]. J Virol Methods, 2008, 147(1): 188-193. doi: 10.1016/j.jviromet.2007.08.023 [20] Poon L L, Leung C S, Tashiro M, et al. Rapid detection of the severe acute respiratory syndrome (SARS) coronavirus by a loop-mediated isothermal amplification assay[J]. Clin Chem, 2004, 50(6): 1050-1052. doi: 10.1373/clinchem.2004.032011 [21] Shiro F, Mizukami Y, Ishida A, et al. Real-time loop-mediated isothermal amplification for the CaMV-35S promoter as a screening method for genetically modified organisms[J]. Eur Food Res Technol, 2004, 218: 496-500. doi: 10.1007/s00217-003-0862-5 [22] Alhassan A, Li Z, Poole C B, et al. Expanding the MDx toolbox for filarial diagnosis and surveillance[J]. Trends in Parasitology, 2015, 31(8): 391-400. doi: 10.1016/j.pt.2015.04.006 [23] Yu L, Wu S, Hao X, et al. Rapid colorimetric detection of COVID-19 coronavirus using a reverse tran-scriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic plat-form: iLACO[J]. medRxiv, 2020: 2020.02. 20.20025874. [24] Laura E L, Sarah N B, Elijah W, et al. Rapid detection of novel coronavirus/Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by reverse transcription loop mediated isothermal amplification[J]. PLoS One. 2020, 15(6): e023468. -


 作者投稿
作者投稿 专家审稿
专家审稿 编辑办公
编辑办公


 下载:
下载: